Caffeoylmalic acid
 | |
| Names | |
|---|---|
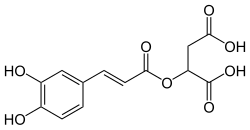
| IUPAC name 2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxybutanedioic acid | |
| Other names (+)-(E)-caffeoyl-L-malic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C13H12O8 |
| Molar mass | 296.231 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Caffeoylmalic acid is an ester of caffeic acid and malic acid found in the leaves and flowers of Parietaria officinalis.[1] It is also found in Chelidonium majus[2] and Urtica dioica.[3]
References
- ^ Budzianowski J (January 1990). "Caffeoylmalic and two pyrrole acids from Parietaria officinalis". Phytochemistry. 29 (10): 3299–301. doi:10.1016/0031-9422(90)80203-S.
- ^ Hahn R, Nahrstedt A (February 1993). "Hydroxycinnamic Acid Derivatives, Caffeoylmalic and New Caffeoylaldonic Acid Esters, from Chelidonium majus". Planta Medica. 59 (1): 71–5. doi:10.1055/s-2006-959608. PMID 17230338.
- ^ Farahpour, MR (January 2015). "Antinociceptive and anti-inflammatory activities of hydroethanolic extract of Urtica dioica" (PDF). Int. J. Biol. Pharm. Allied Sci. 4 (1): 165–167 – via Google Scholar.
- v
- t
- e
Types of hydroxycinnamic acids
| Precursor |
|
|---|---|
| Monohydroxycinnamic acids (Coumaric acids) |
|
| Dihydroxycinnamic acids |
|
| Trihydroxycinnamic acids |
|
| O-methylated forms | |
| others |
| glycoside-likes |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tartaric acid esters |
| ||||||||
| Other esters with caffeic acid |
| ||||||||
| Caffeoyl phenylethanoid glycoside (CPG) |
|
| Dimers |
|
|---|---|
| Trimers |
|
| Tetramers |
|
coenzyme A (CoA)
- Caffeoyl-CoA
- Cinnamoyl-CoA
- Coumaroyl-CoA
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











