Xanthorhamnin
 | |
| Names | |
|---|---|
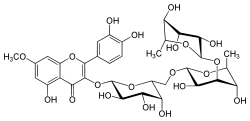
| IUPAC name 3′,4′,5-Trihydroxy-7-methoxy-2-[α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→6)-β-D-galactopyranosyloxy]flavone | |
| Systematic IUPAC name (42S,43R,44S,45R,46R,72R,73R,74R,75S,76S,92S,93R,94R,95R,96S)-13,14,25,43,44,45,73,75,93,94,95-Undecahydroxy-27-methoxy-76,96-dimethyl-24H-3,6,8-trioxa-2(2,3)-[1]benzopyrana-4(2,6),7(2,4),9(2)-tris(oxana)-1(1)-benzenanonaphan-24-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C34H42O20 |
| Molar mass | 770.68 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Xanthorhamnin is a chemical compound. It can be isolated from buckthorn berries (Rhamnus catharticus).[1]
The aglycone of xanthorhamnin is rhamnetin.
References
- ^ "The Color of Art Pigment Database: Pigment Yellow, PY". Art is Creation.
- v
- t
- e
Flavonols and their conjugates
| Aglycones |
|
|---|
| Aglycones |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjugates |
|
| Aglycones |
| ||||
|---|---|---|---|---|---|
| Glycosides |
|
| Aglycones |
|
|---|---|
| Glycosides |
|
| Aglycones |
|---|
| Aglycones | |
|---|---|
| Glycosides |
|
| Glycosides |
|---|
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











